m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways
Authors
10-11-2017
12:00pm
PST
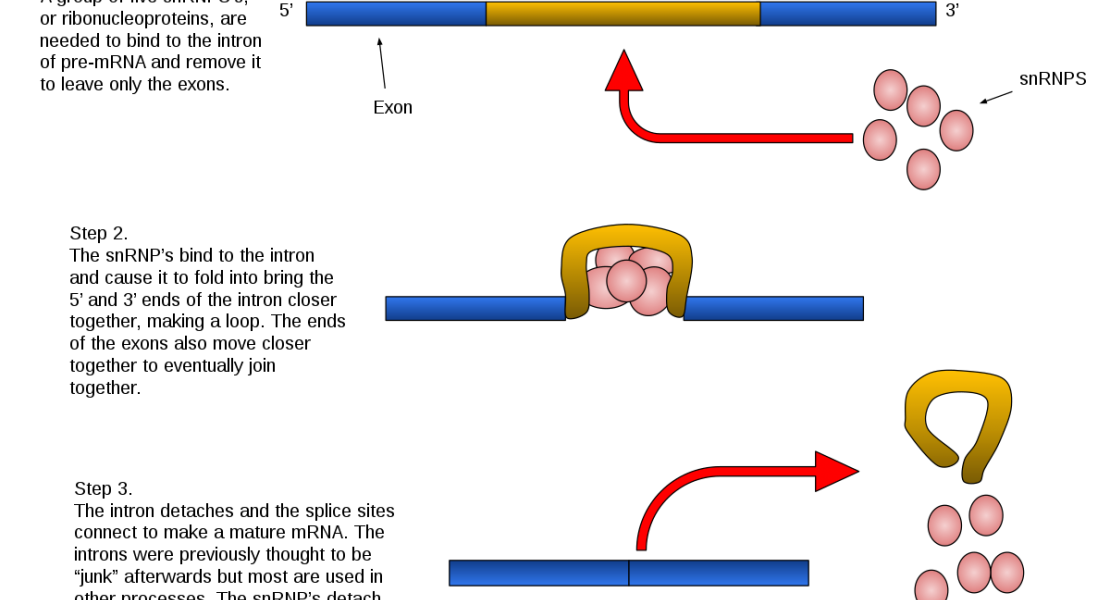
Abstract
N6 -methyladenosine (m6 A) is the most common and abundant messenger RNA modification, modulated by ‘writers’, ‘erasers’ and ‘readers’ of this mark1,2 . In vitro data have shown that m6 A influences all fundamental aspects of mRNA metabolism, mainly mRNA stability, to determine stem cell fates3,4 . However, its in vivo physiological function in mammals and adult mammalian cells is still unknown. Here we show that the deletion of m6 A ‘writer’ protein METTL3 in mouse T cells disrupts T cell homeostasis and differentiation. In a lymphopaenic mouse adoptive transfer model, naive Mettl3-deficient T cells failed to undergo homeostatic expansion and remained in the naive state for up to 12 weeks, thereby preventing colitis. Consistent with these observations, the mRNAs of SOCS family genes encoding the STAT signalling inhibitory proteins SOCS1, SOCS3 and CISH were marked by m6 A, exhibited slower mRNA decay and showed increased mRNAs and levels of protein expression in Mettl3-deficient naive T cells. This increased SOCS family activity consequently inhibited IL7-mediated STAT5 activation and T cell homeostatic proliferation and differentiation. We also found that m6 A has important roles for inducible degradation of Socs mRNAs in response to IL-7 signalling in order to reprogram naive T cells for proliferation and differentiation. Our study elucidates for the first time, to our knowledge, the in vivo biological role of m6 A modification in T-cell-mediated pathogenesis and reveals a novel mechanism of T cell homeostasis and signaldependent induction of mRNA degradation.
