Intragenic DNA methylation prevents spurious transcription initiation
Authors
04-05-2017
12:00pm
PST
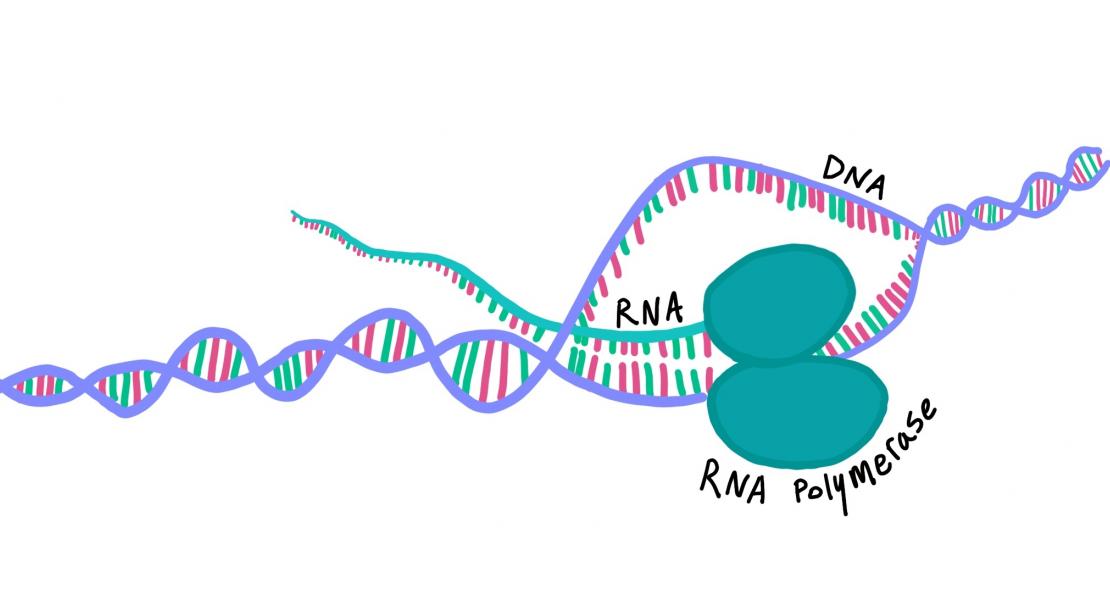
Abstract
In mammals, DNA methylation occurs mainly at CpG dinucleotides. Methylation of the promoter suppresses gene expression, but the functional role of gene-body DNA methylation in highly expressed genes has yet to be clarified. Here we show that, in mouse embryonic stem cells, Dnmt3b-dependent intragenic DNA methylation protects the gene body from spurious RNA polymerase II entry and cryptic transcription initiation. Using different genome-wide approaches, we demonstrate that this Dnmt3b function is dependent on its enzymatic activity and recruitment to the gene body by H3K36me3. Furthermore, the spurious transcripts can either be degraded by the RNA exosome complex or capped, polyadenylated, and delivered to the ribosome to produce aberrant proteins. Elongating RNA polymerase II therefore triggers an epigenetic crosstalk mechanism that involves SetD2, H3K36me3, Dnmt3b and DNA methylation to ensure the fidelity of gene transcription initiation, with implications for intragenic hypomethylation in cancer.
