Endogenous siRNAs and noncoding RNA-derived small RNAs are expressed in adult mouse hippocampus and are up-regulated in olfactory discrimination training.
Abstract
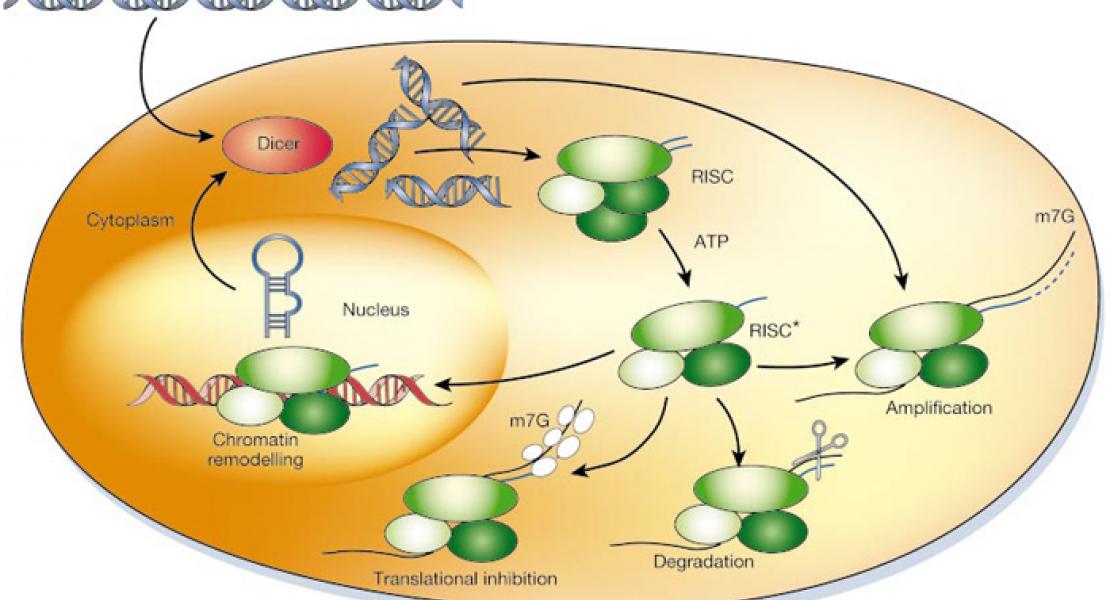
We previously proposed that endogenous siRNAs may regulate synaptic plasticity and long-term gene expression in the mammalian brain. Here, a hippocampal-dependent task was employed in which adult mice were trained to execute a nose-poke in a port containing one of two simultaneously present odors in order to obtain a reward. Mice demonstrating olfactory discrimination training were compared to pseudo-training and nose-poke control groups; size-selected hippocampal RNA was subjected to Illumina deep sequencing. Sequences that aligned uniquely and exactly to the genome without uncertain nucleotide assignments, within exons or introns of MGI annotated genes, were examined further. The data confirm that small RNAs having features of endogenous siRNAs are expressed in brain; that many of them derive from genes that regulate synaptic plasticity (and have been implicated in neuropsychiatric diseases); and that hairpin-derived endo-siRNAs and the 20- to 23-nt size class of small RNAs show a significant increase during an early stage of training. The most abundant putative siRNAs arose from an intronic inverted repeat within the SynGAP1 locus; this inverted repeat was a substrate for dicer in vitro, and SynGAP1 siRNA was specifically associated with Argonaute proteins in vivo. Unexpectedly, a dramatic increase with training (more than 100-fold) was observed for a class of 25- to 30-nt small RNAs derived from specific sites within snoRNAs and abundant noncoding RNAs (Y1 RNA, RNA component of mitochondrial RNAse P, 28S rRNA, and 18S rRNA). Further studies are warranted to characterize the role(s) played by endogenous siRNAs and noncoding RNA-derived small RNAs in learning and memory.
