EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2
Abstract
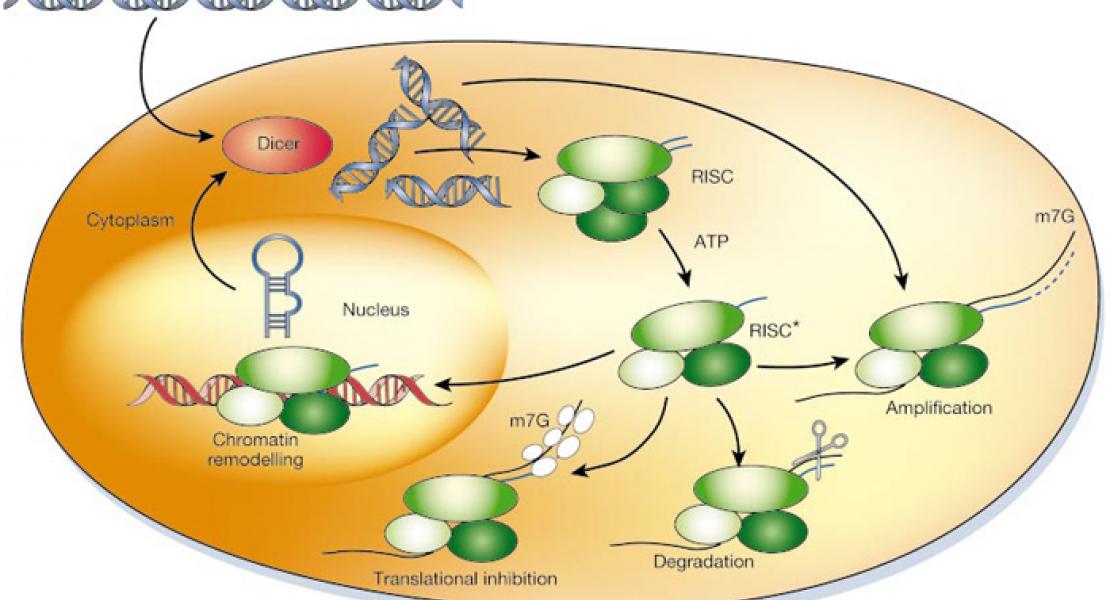
MicroRNAs (miRNAs) are generated by two-step processing to yield small RNAs that negatively regulate target gene expression at the post-transcriptional level1 . Deregulation of miRNAs has been linked to diverse pathological processes, including cancer2,3. Recent studies have also implicated miRNAs in the regulation of cellular response to a spectrum of stresses4 , such as hypoxia, which is frequently encounteredin the poorly angiogenic core of a solid tumour5 . However, the upstream regulators of miRNA biogenesis machineries remain obscure, raising the question of how tumour cells efficiently coordinate and impose specificity on miRNA expression and function in response to stresses. Here we show that epidermal growth factor receptor (EGFR), which is the product of a well-characterized oncogene in human cancers, suppresses the maturation of specific tumour-suppressor-like miRNAs in response to hypoxic stress through phosphorylation of argonaute 2 (AGO2) at Tyr 393. The association between EGFR andAGO2is enhanced by hypoxia, leading to elevated AGO2-Y393 phosphorylation, which in turn reduces the binding of Dicer to AGO2 and inhibits miRNA processing from precursor miRNAs to mature miRNAs. We also identify a long-loop structure in precursor miRNAs as a critical regulatory element in phosphoY393-AGO2-mediated miRNA maturation. Furthermore, AGO2- Y393 phosphorylation mediates EGFR-enhanced cell survival and invasiveness under hypoxia, and correlates with poorer overall survival in breast cancer patients. Our study reveals a previously unrecognized function of EGFR in miRNA maturation and demonstrates how EGFR is likely to function as a regulator of AGO2 through novel post-translational modification. These findings suggest that modulation of miRNA biogenesis is important for stress response in tumour cells and has potential clinical implications.
