Degradation of Stop Codon Read-through Mutant Proteins via the Ubiquitin-Proteasome System Causes Hereditary Disorders
Authors
10-26-2016
12:00pm
PST
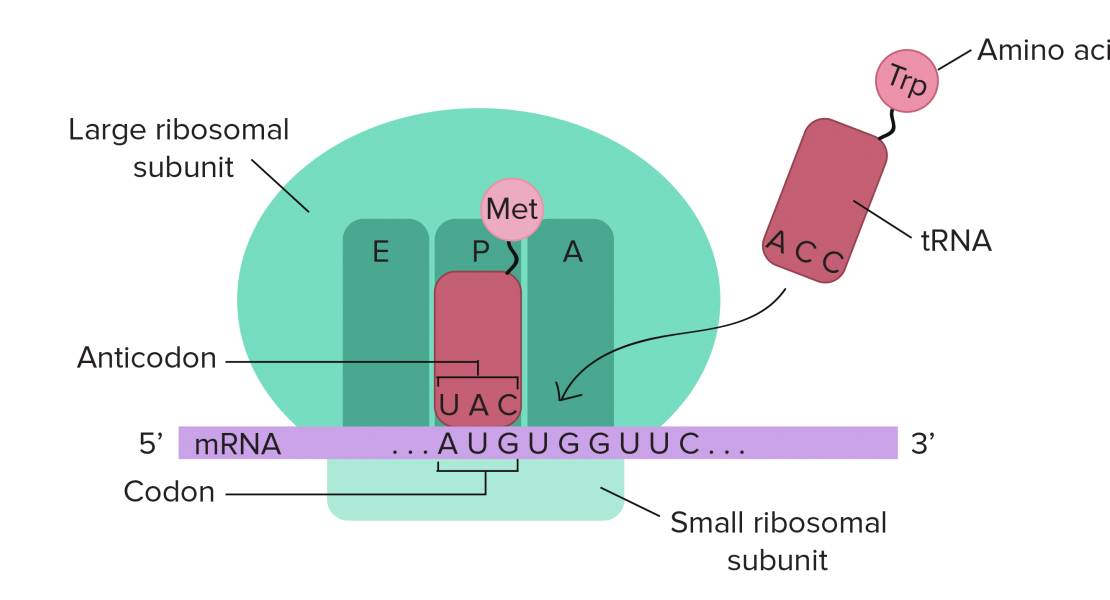
Abstract
During translation, stop codon read-through occasionally happens when the stop codon is misread, skipped, or mutated, resulting in the production of aberrant proteins with C-terminal extension. These extended proteins are potentially deleterious, but their regulation is poorly understood. Here we show in vitro and in vivo evidence that mouse cFLIP-L with a 46-amino acid extension encoded by a read-through mutant gene is rapidly degraded by the ubiquitin-proteasome system, causing hepatocyte apoptosis during embryogenesis. The extended peptide interacts with an E3 ubiquitin ligase, TRIM21, to induce ubiquitylation of the mutant protein. In humans, 20 read-through mutations are related to hereditary disorders, and extended peptides found in human PNPO and HSD3B2 similarly destabilize these proteins, involving TRIM21 for PNPO degradation. Our findings indicate that degradation of aberrant proteins with C-terminal extension encoded by read-through mutant genes is a mechanism for loss of function resulting in hereditary disorders.
